Introduction to L7|ESP and L7 MES
How does L7|ESP create a unified platform?
L7|ESP combines the digitization of data and the digitalization of processes to contextualize data and unify business value chains.
Digitization – the conversion of analog information to digital (paper to electronic) and automation of existing manual and paper-based processes.
Digitalization – the use of digital technologies and digitized data to impact how work gets done and transform how departments within a company interact, and how the company interacts with its value chain partners.
L7|ESP tackles complex business operations by creating building blocks that can be reused and extended to various applications.
The Entity is the smallest data component, it represents any resource that is submitted to, or a component of, a business process (e.g., Batch, equipment, intermediate sample).
The Protocol is the smallest process component, it defines the data to collect while processing the Batch (e.g., equipment check, cell count, media change).
Protocols incorporate L7|ESP’s data models (Entity Types, Container Types, Item Types, and Vendors) to unify the value chain, providing End Users with access to sample, location, and inventory management in one interface.
Ultimately Protocols are grouped into Workflows, and Workflows are connected into Workflow Chains to digitalize business processes.
The Digital Transformation in Precision Therapeutics
The industry’s heavy reliance on paper-based processes has created paper silos that:
Inhibit collaboration and process improvement.
Reduce understanding and insight.
Drastically slow delivery.
Tie up talent in reviews, corrections, and investigations.
The characterization, manufacturing, and control (CMC) process must change to incorporate learnings from development to manufacturing, to better understand critical process parameters (CPPs) and the critical quality attributes (CQAs) they affect.
Data becomes the product when process improvements lead to better patient outcomes.
A Manufacturing Execution System (L7 MES) as part of a unified platform (L7|ESP) automates a business process and captures contextualized data where people and equipment meet the process. Automated quality by design streamlines the review process with “review by exception” and supports a right-first-time, every-time manufacturing environment.
Digitalization leads to better products at faster speeds, and it all starts with L7 MES in L7|ESP.
L7 MES Landing Page
The L7 MES landing page displays all Batches that have been created in L7 MES. To review or work with a Batch, select the relevant Batch Number.
Batches are listed by default in descending order based on their creation date, and organized into three (3) tabs:
In Process – Batches that are currently active (default view).
Completed – Batches that have been completed or failed.
All – in-process and completed Batches.
All three (3) tabs display the same Batch details:
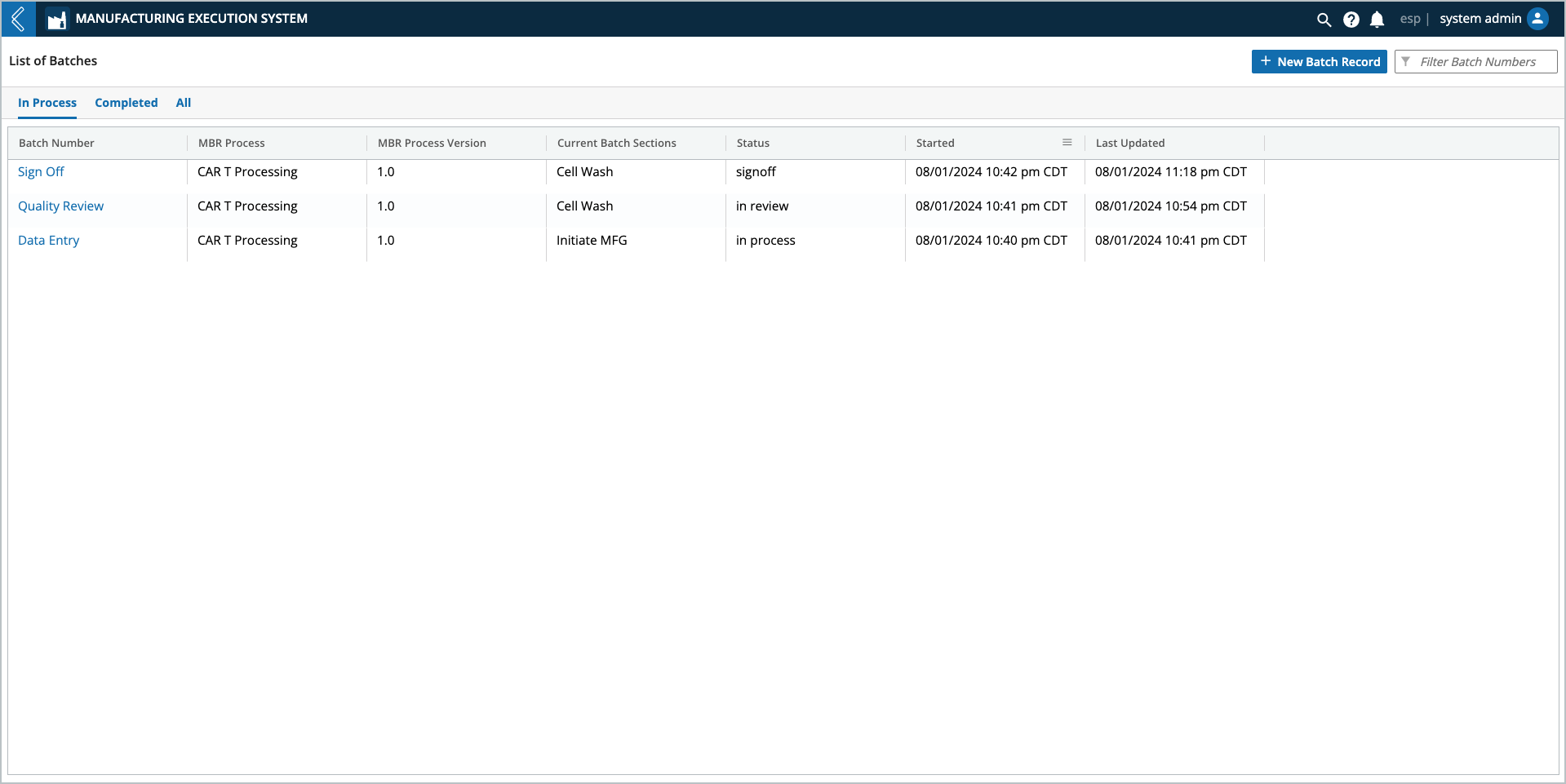
Batch Number – name of the Batch.
MBR Process – Recipe used for the Batch.
MBR Process Version – Recipe version used for the Batch.
Current Batch Sections:
If the Batch is in process, this column lists all active Batch Sections.
If the Batch is completed, this column lists the last Batch Section.
If the Batch is failed, this column lists the last Batch Section that was active at the time of failure.
Status:
In process – data is actively being collected.
In review – all Sections and Steps have been completed, but at least one (1) Step requires review.
Reviewed – all Sections and Steps have been reviewed, and the Batch is ready for quality review.
Sign-off – quality review signatures are complete, and the Batch is ready for final sign-off.
Complete – all work for the Batch is complete.
Failed – the Batch has been failed.
Started – date time the Batch was created.
Last Update – last date time any activity was recorded for the Batch.